Check our M-Series (Metallic) and D-Series (Ceramic) catalytic converters designed especially for diesel engines…
How Does an Oxidation Catalyst Work?
The hydrocarbon emissions from LPG engines will contain a mixture of propane, butane, ethane, and other compounds. Both CO and hydrocarbons are converted in the oxidation catalyst to carbon dioxide and water vapor which are non-toxic gases. The conversion of CO and HC in the catalyst requires oxygen, as shown in the reaction equations. Usually, there is not enough oxygen in the exhaust gases of LPG engines to burn all of the pollutants. Oxidation catalyst systems frequently require that extra air, called secondary air, be introduced into the exhaust system in front of the catalyst.
Typical conversion efficiencies for carbon monoxide and various hydrocarbons (butane, propane, and methane) in the Nett® oxidation catalyst are shown in Figure 8. Catalyst activity increases with temperature. A minimum exhaust temperature of about 200°C is necessary for the catalyst to "light-off". Higher temperatures are necessary for hydrocarbon conversion. LPG exhaust contains short carbon chain hydrocarbons which are more difficult to convert in the catalyst than those found in diesel or gasoline exhaust. As illustrated in the graph, the shorter the carbon chain the higher the conversion temperature.
Nett Technologies’ Diesel Oxidation Catalysts (DOC)

Request A Quote
Photo Gallery

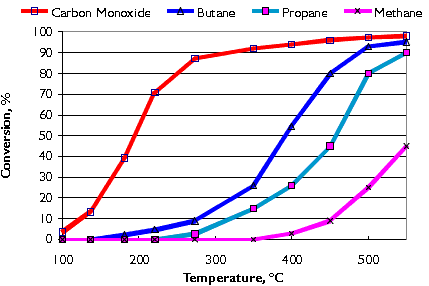 Figure 8. Conversion of CO and Hydrocarbons in Oxidation Catalyst
Figure 8. Conversion of CO and Hydrocarbons in Oxidation Catalyst